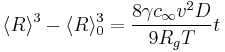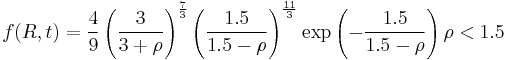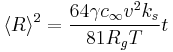Ostwald ripening
Ostwald ripening is an observed phenomenon in solid solutions or liquid sols which describes the change of an inhomogeneous structure over time. In other words, over time, small crystals or sol particles dissolve, and redeposit onto larger crystals or sol particles.[1]
Dissolution of small crystals or sol particles and the redeposition of the dissolved species on the surfaces of larger crystals or sol particles was first described by Wilhelm Ostwald in 1896.[2] Ostwald ripening is generally found in water-in-oil emulsions, while flocculation is found in oil-in-water emulsions [3]
Contents |
Mechanism
This thermodynamically-driven spontaneous process occurs because larger particles are more energetically favored than smaller particles.[4] This stems from the fact that molecules on the surface of a particle are energetically less stable than the ones in the interior. Consider a cubic crystal of atoms: all the atoms inside are bonded to 6 neighbors and are quite stable, but atoms on the surface are only bonded to 5 neighbors or less, which makes these surface atoms less stable. Large particles are more energetically favorable since, continuing with our example, more atoms are bonded to 6 neighbors and fewer atoms are at the unfavorable surface. As the system tries to lower its overall energy, molecules on the surface of a small particle (energetically unfavorable, with only 3 or 4 or 5 bonded neighbors) will tend to detach from the particle, as per the Kelvin equation, and diffuse into the solution. When all small particles do this, it increases the concentration of free atoms in solution. When the free atoms in solution are supersaturated, the free atoms have a tendency to condense on the surface of larger particles.[4]. Therefore, all smaller particles shrink, while larger particles grow, and overall the average size will increase. After an infinite amount of time, the entire population of particles will have become one, huge, spherical particle to minimize the total surface area.
In 1961, Lifshitz and Slyozov [5] performed a mathematical investigation of Ostwald ripening in the case where diffusion of material is the slowest process. The derivation first states how a single particle grows in a solution, and this equation describes where the boundary is between small, shrinking particles and large, growing particles. Through a lengthy and abstract mathematical derivation, they conclude that the average radius of the particles <R>, grows as follows:
where
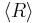 = average radius of all the particles
= average radius of all the particles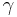 = particle surface tension or surface energy
= particle surface tension or surface energy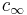 = solubility of the particle material
= solubility of the particle material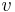 = molar volume of the particle material
= molar volume of the particle material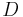 = diffusion coefficient of the particle material
= diffusion coefficient of the particle material = ideal gas constant
= ideal gas constant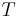 = absolute temperature
= absolute temperature
and
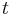 = time
= time
Two things should be noted about this growth law: (a) The quantity 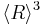 is different from
is different from 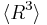 , and only the latter one can be used to calculate average volume, and (b) the statement that
, and only the latter one can be used to calculate average volume, and (b) the statement that 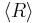 goes as
goes as 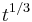 relies on
relies on 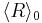 being zero; but because nucleation is a separate process from growth, this places
being zero; but because nucleation is a separate process from growth, this places 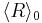 outside the bounds of validity of the equation. In contexts where the actual value of
outside the bounds of validity of the equation. In contexts where the actual value of 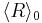 is irrelevant, an approach that respects the meanings of all terms is to take the time derivative of the equation to eliminate
is irrelevant, an approach that respects the meanings of all terms is to take the time derivative of the equation to eliminate 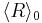 and
and 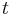 . Another such approach is to change the
. Another such approach is to change the 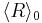 to
to 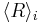 with the initial time
with the initial time 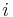 having a positive value.
having a positive value.
Also contained in the Lifshitz and Slyozov derivation is an equation for the size distribution function 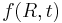 of particles. For convenience, the radius of particles is divided by the average radius to form a new variable,
of particles. For convenience, the radius of particles is divided by the average radius to form a new variable, 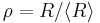 :
:
Ironically, at the same time as Lifshitz and Slyozov published their findings, Carl Wagner performed his own mathematical investigation of Ostwald ripening [6], examining both systems where diffusion was slow and also where attachment and detachment at the particle surface was slow. Although his calculations and approach were different, Wagner made exactly the same conclusions as Lifshitz and Slyozov for slow-diffusion systems. This duplicate derivation went unnoticed for years because the two scientific papers were published on opposite sides of the Iron Curtain in 1961. It was not until 1975 that Kahlweit addressed the fact that the theories were identical [7] and combined them into the Lifshitz-Slyozov-Wagner or LSW Theory of Ostwald ripening. Many experiments and simulations have proven LSW theory to be robust and accurate. Even some systems that undergo spinodal decomposition have been shown to quantitatively obey LSW theory after initial stages of growth [8].
For the curious, Wagner derived that when attachment and detachment of molecules is slower than diffusion, then the growth rate becomes
where 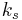 is the reaction rate constant of attachment with units of length per time. Since the average radius is usually something that can be measured in experiments, it is fairly easy to tell if a system is obeying the slow-diffusion equation or the slow-attachment equation. If, of course, the experimental data obeys neither equation, then it is likely that another mechanism is taking place and Ostwald ripening is not occurring.
is the reaction rate constant of attachment with units of length per time. Since the average radius is usually something that can be measured in experiments, it is fairly easy to tell if a system is obeying the slow-diffusion equation or the slow-attachment equation. If, of course, the experimental data obeys neither equation, then it is likely that another mechanism is taking place and Ostwald ripening is not occurring.
Although LSW theory and Ostwald ripening were intended for solids ripening in a fluid, Ostwald ripening is also observed in liquid-liquid systems. For example, in an oil-in-water emulsion polymerization,[3] In this case, Ostwald ripening causes the diffusion of monomers (i.e. individual molecules or atoms) from smaller droplets to larger droplets due to greater solubility of the single monomer molecules in the larger monomer droplets. The rate of this diffusion process is linked to the solubility of the monomer in the continuous (water) phase of the emulsion. This can lead to the destabilization of emulsions (for example, by creaming and sedimentation).[9]
Specific examples
An everyday example of Ostwald ripening is the re-crystallization of water within ice cream which gives old ice cream a gritty, crunchy texture. Larger ice crystals grow at the expense of smaller ones within the ice cream, thereby creating a coarser texture.[10]
Another gastronomical example is in the ouzo effect, where the droplets in the cloudy microemulsion grow by Ostwald ripening.
In geology, it is the textural coarsening, aging or growth of phenocrysts and crystals in solid rock which is below the solidus temperature. It is often ascribed as a process in the formation of orthoclase megacrysts,[11] as an alternative to the physical processes governing crystal growth from nucleation and growth rate thermochemical limitations.
In chemistry, the term refers to the growth of larger crystals from those of smaller size which have a higher solubility than the larger ones. In the process, many small crystals formed initially slowly disappear, except for a few that grow larger, at the expense of the small crystals. The smaller crystals act as fuel for the growth of bigger crystals. Limiting Ostwald ripening is fundamental in modern technology for the solution synthesis of quantum dots.[12] Ostwald ripening is also the key process in the digestion of precipitates, an important step in gravimetric analysis. The digested precipitate is generally purer, and easier to wash and filter.
Ostwald ripening can also occur in emulsion systems, with molecules diffusing from small droplets to large ones through the continuous phase. When a miniemulsion is desired, an extremely hydrophobic compound is added to stop this process from taking place.
See also
- Rock microstructure
- Kelvin equation
- Kirkendall effect
- Critical radius
- Solubility_equilibrium#Particle_size_effect
- Flocculation
- Aggregation
- Coalescence
References
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "Ostwald ripening".
- ^ W. Ostwald. 1896. Lehrbuch der Allgemeinen Chemie, vol. 2, part 1. Leipzig, Germany.
- ^ a b Hubbard, Arthur T. (2004). Encyclopedia of Surface and Colloid Science. CRC Press. p. 4230. ISBN 0824707591. http://books.google.com/?id=vnb2X7Q8_cYC&pg=PA4230&lpg=PA4230&dq=ostwald+ripening+emulsion+polymerization. Retrieved 2007-11-13.
- ^ a b Ratke, Lorenz; Voorhees, Peter W. (2002). Growth and Coarsening: Ostwald Ripening in Material Processing. Springer. pp. 117–118. ISBN 3540425632. http://books.google.com/?id=baKRnEuSBXkC&dq=ostwald+ripening&printsec=frontcover. Retrieved 2007-11-15.
- ^ I.M. Lifshitz, V.V. Slyozov (1961). "The Kinetics of Precipitation from Supersaturated Solid Solutions". Journal of Physics and Chemistry of Solids 19 (1–2): 35–50. Bibcode 1961JPCS...19...35L. doi:10.1016/0022-3697(61)90054-3.
- ^ C. Wagner (1961). "THEORIE DER ALTERUNG VON NIEDERSCHLAGEN DURCH UMLOSEN (OSTWALD-REIFUNG)". Zeitschrift fur Elektrochemie 65 (7): 581–591.
- ^ M. Kahlweit (1975). "Ostwald Ripening of Precipitates". Advances in Colloid and Interface Science 5 (1): 1–35. doi:10.1016/0001-8686(75)85001-9.
- ^ N. Vladimirova, A. Malagoli, R. Mauri (1998). "Diffusion-driven phase separation of deeply quenched mixtures". Physical Review E 58 (6): 7691–7699. Bibcode 1998PhRvE..58.7691V. doi:10.1103/PhysRevE.58.7691.
- ^ Branen, Alfred Larry (2002). Food Additives. CRC Press. p. 724. ISBN 0824793439. http://books.google.com/?id=K8urEPJvXNsC&pg=PA724&lpg=PA724&dq=ostwald+ripening+destabilization+emulsion. Retrieved 2007-11-15.
- ^ Clark, Chris (2004). The Science of Ice Cream. Royal Society of Chemistry. pp. 78–79. ISBN 0854046291. http://books.google.com/?id=bKZ1oICZWywC&pg=PA78&lpg=PA78&dq=ice+cream+ostwald. Retrieved 2007-11-13.
- ^ Mock, A. (2003). "Using Quantitative Textural Analysis to Understand the Emplacement of Shallow-Level Rhyolitic Laccoliths—a Case Study from the Halle Volcanic Complex, Germany". Journal of Petrology 44 (5): Pp. 833–849. doi:10.1093/petrology/44.5.833. http://petrology.oxfordjournals.org/cgi/content/full/44/5/833. Retrieved 2007-11-14.
- ^ Vengrenovich, R.D.; Gudyma, Yu. V.; Yarema, S. V. (December 2001). "Ostwald ripening of quantum-dot nanostructures". Semiconductors 35 (12): pp.1378–1382. Bibcode 2001Semic..35.1378V. doi:10.1134/1.1427975. http://www.springerlink.com/content/814551gg47485391/. Retrieved 2007-11-14.
External links
- Ostwald Ripening a 3D Kinetic Monte Carlo simulation
|
||||||||||||||||